Take Action at the Cancer Center
The Clinical Trials Office provides study management services to UIC faculty conducting clinical research related to the prevention, diagnosis, or treatment of cancer.
Our services include:
- Management of the entire study start-up process including:
- Budget development & negotiation
- Protocol Review Committee (PRC) submission
- IRB Submission
- Routing for contract negotiation and UIC hospital approvals
- Clinical research coordination
- Data management
- IRB submissions
- FDA submissions
- Study financial management
- Quality assurance monitoring
- Staff training and education
To start-up a study, contact the CTO Start-Up Specialist at [email protected].
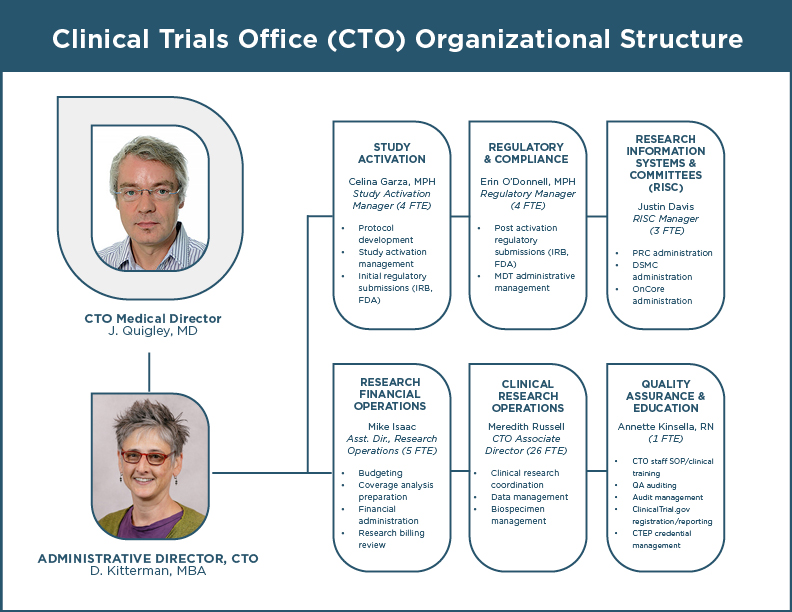
Organizational Chart
Disease Teams
The University of Illinois Cancer Center Protocol Review and Monitoring System ensures that all studies performed at University of Illinois Cancer Center are safe, appropriate for the population we serve, and aligned with our research priorities.
Data Safety Monitoring Committee (DSMC)
The University of Illinois Cancer Center Protocol Review and Monitoring System ensures that all studies performed at University of Illinois Cancer Center are safe, appropriate for the population we serve, and aligned with our research priorities.
Clinical Trials Office Personnel
| Name | Position | Phone number | |
|---|---|---|---|
| Bratslavsky, Sasha | Clinical Research Data Coordinator | [email protected] | |
| Broccolo, Rose | Regulatory Specialist | [email protected] | |
| D’Ambrosia, Kristin | Study Start Up Specialist | [email protected] | |
| Danmole, Mopelola | Clinical Research Coordinator | [email protected] | |
| Davis, Justin | Research Systems and Committees Manager | 312-996-9707 | [email protected] |
| Dudey, Abhishek | Study Start Up Specialist | [email protected] | |
| Garza, Celina | Senior Regulatory Specialist, Start Up | 312-996-8866 | [email protected] |
| Genio Goluch, Ria | Clinical Research Finance Manager | 312-413-5883 | [email protected] |
| Giwa, Latifat | Regulatory Specialist | [email protected] | |
| Gordillo, Liliana | Clinical Research Data Coordinator | [email protected] | |
| Gu, Ethan | Clinical Research Coordinator | [email protected] | |
| Guptan, Raj | Regulatory Specialist | [email protected] | |
| Guzman, Arielle | Senior Clinical Research Coordinator | 312-355-8838 | [email protected] |
| Isaac, Michael | Assistant Director, Research Services | 312-413-5630 | [email protected] |
| Karan, Michelle | Clinical Research Coordinator | [email protected] | |
| Kassie, Rahel | Clinical Research Coordinator | 312-413-4262 | [email protected] |
| Kinsella, Annette | Quality Assurance and Education Specialist | 312-996-5931 | [email protected] |
| Kitsch, Kristen | Senior Clinical Research Coordinator | 312-355-5767 | [email protected] |
| Kitterman, Darlene | Administrative Director | 312-996-9272 | [email protected] |
| Mantice, Maria | Clinical Research Data Coordinator | 312-355-1472 | [email protected] |
| Myrick, Tashay | Financial Analyst | 312-996-4930 | [email protected] |
| Noreen, Sobia | Protocol Writer | [email protected] | |
| O’Donnell, Erin | Regulatory Specialist | 312-996-9913 | [email protected] |
| Patel, Radhika | Research Systems and Committees Coordinator | 312-996-9071 | [email protected] |
| Puz, Sarah | Clinical Research Data Coordinator | 312-996-6750 | [email protected] |
| Qazi, Omer | Clinical Research Coordinator | [email protected] | |
| Rosa, Carmen | Financial Analyst | 312-996-0140 | [email protected] |
| Russell, Meredith | Associate Director | 312-355-5112 | [email protected] |
| Ryan, Tara | Clinical Research Coordinator | [email protected] | |
| Rygalski, Kayleigh | Senior Clinical Research Data Coordinator | 312-355-2090 | [email protected] |
| Sane, Nitin | Clinical Research Coordinator | 312-355-0741 | [email protected] |
| Sanidad, Andrew | Clinical Research Data Coordinator | 312-413-0433 | [email protected] |
| Secreto, Alyssa | Senior Research Specialist | [email protected] | |
| Shi, Richard | Senior Clinical Research Coordinator | 312-996-9749 | [email protected] |
| Strange, Meghan | Senior Clinical Research Data Coordinator | 312-996-6676 | [email protected] |
| Toh, Roxana | Regulatory Specialist | 312-996-2078 | [email protected] |
| Vega, Marisol | Clinical Research Data Coordinator | 312-355-5035 | [email protected] |
| Villareal, Elyssa | Research Systems and Committees | [email protected] | |
| Yin, Ruihong | Clinical Research Coordinator | [email protected] |
Contact Us
Contact the University of Illinois Cancer Center Clinical Trials Office at:
