
Researchers from the University of Illinois Cancer Center found that ethnic and racial minorities, the elderly and women are significantly underrepresented in clinical trials for oncology drugs, limiting the usefulness of clinical trial data for physicians and their patients.
The study abstract was published today in advance of the American Society of Clinical Oncology (ASCO) Annual Meeting, scheduled for June 4-8. Authors Ryan Nguyen, DO, Yomaira Silva, MS-3, and VK Gadi, MD, PhD, analyzed data from pivotal clinical trials that were used to justify U.S. Food and Drug Administration approval of new drugs to treat breast, colorectal, lung and prostate cancer.
The FDA granted novel or new use approval to 60 drugs during the 12-year period spanning 2008-2020. Compared to the U.S. cancer population, the researchers found underrepresentation of Black, Hispanic, elderly, and female patients in the pivotal clinical trials used to support approval of the new drugs. Equitable representation in cancer clinical trials will help researchers understand how a patient’s race, age, ethnicity and gender can impact the efficacy and toxicity of oncology drugs, according to Nguyen.
“Every day I talk to patients about potential treatments for their cancer and in looking at the data, the populations from the clinical trials rarely reflects my patient population, which is a concern,” Nguyen said. “In one instance, over five hundred women participated in a clinical trial for a new breast cancer drug and only eight of them were Black. Once we started looking through trial enrollment, we noticed lack of adequate representation was a recurring pattern.”
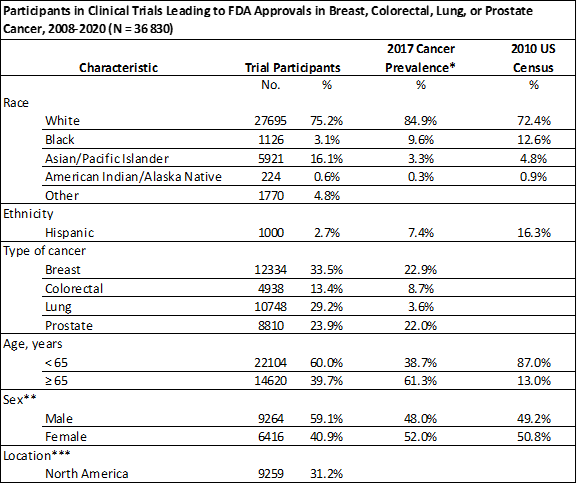
Global pharmaceutical companies often take a multinational approach to clinical trials for promising cancer treatments, Nguyen explained, which is a reason the demographics of the patients in the trials may not align with the population of the United States. The researchers also found inconsistent reporting of racial demographics, which were reported in 78% of manuscripts, 68% of pages on ClinicalTrials.gov and 98% of FDA labels or approval summaries.
The disparities were not limited to race and ethnicity. Elderly patients account for more than 61% of cancers, yet were significantly less likely to be enrolled in a clinical trial. Female patients were unrepresented in colorectal and lung cancer trials.
Numerous factors contribute to a lack of adequate representation in cancer clinical trials, Nguyen added, including a lack of consistent demographic reporting, trials preferentially being in more affluent areas, provider bias and patient mistrust of clinical trials.
